By Caribbean Medical News Staff
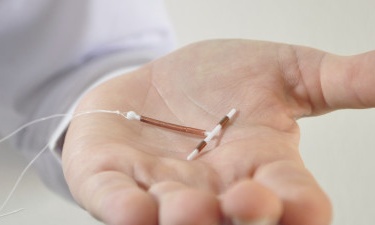
The US Food and Drug Administration (FDA) approved a new intrauterine device (IUD), Liletta that is as effective as sterilization but gives women the option to reverse the effects and become pregnant if they want to. The (IUD) is a T-shaped piece of plastic that is inserted into the uterus to prevent fertilization. Liletta releases the hormone levonorgestrel to prevent thickening of the womb lining, inhibiting pregnancy for up to three years.
The hormonal IUDs are slightly more effective than commonly used copper IUDs such as Teva Pharmaceutical Industry Ltd’s Paragard, which last longer but provide no control of menstrual blood flow. While other devices offer between three and 12 years of protection, Liletta is approved for use for up to three years.
Michael WaterHouse, an analyst at investment research firm Morningstar, said he expected peak global sales of below $500m. The device will be competing with Bayer AG’s hormonal IUDs Mirena and Skyla in the United States.
Liletta is being tested in the largest-ever US trial for IUDs, with a diverse patient population encompassing a range of weights, ages and races. It also includes women who have given birth as well as those who have not. The trial is evaluating Liletta’s use for up to seven years.
According to the US Centers for Disease Control (CDC) long-acting reversible contraceptives (LARCs), including IUDs and implants, are more effective than other contraceptives such as pills and patches and are nearly as effective as sterilization.